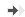Newsletter # 78

Animal models
In the preclinical setting, sensitive and reproducible behavioral screening paradigms need to be established since the ability of ketamine to affect the depressive-like behavior in rats and mice is not replicated consistently from one lab to the other.
-
-
■ A clear separation between the marble burying and the locomotor activity is observed in the combined procedure.
■ This separation is critical for the identification of antidepressant in the marble burying paradigm.
Get in touch


 PREVIOUS
PREVIOUS